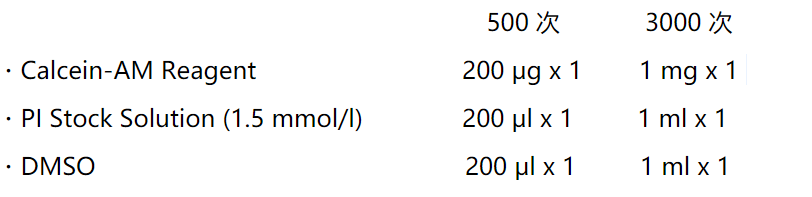产品解说
活动进行中
订购满5000元,200元礼品等你拿
凑单关联产品TOP5
NO.1. Cell Counting Kit-8 细胞增殖毒性检测
NO.2. Annexin V, FITC Apoptosis Detection Kit 细胞凋亡检测
NO.3. FerroOrange 细胞亚铁离子检测
NO.4. Liperfluo 细胞脂质过氧化物检测
NO.5. ROS Assay Kit 活性氧检测
试剂盒内含
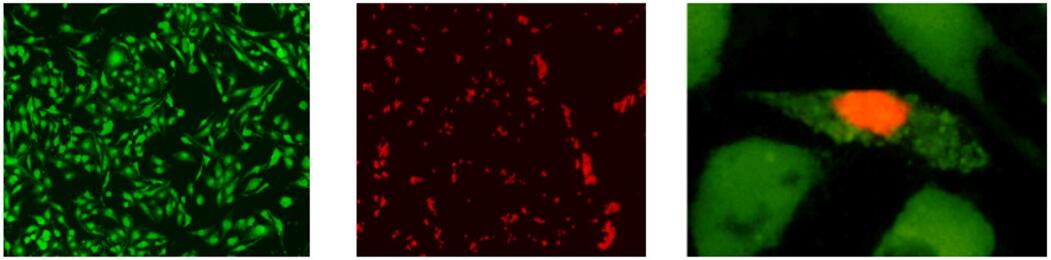
500 次 3000 次
・Calcein-AM Reagent 200 μg x 1 1 mg x 1
・PI Stock Solution (1.5 mmol/l) 200 μl x 1 1 ml x 1
・DMSO 200 μl x 1 1 ml x 1
产品概述
Calcein-AM/PI细胞双染试剂盒内含两种染料:Calcein-AM和Propidium Iodide (PI)。这个试剂盒可在荧光显微镜下同时观察在同一个细胞培养皿中的活细胞和死细胞。Calcein-AM可透过细胞膜,通过活细胞内的酯酶作用脱去AM基团,产生的Calcein (钙黄绿素) 发出强绿色荧光,因此活细胞在荧光显微镜下可被检测到绿色荧光。另一方面PI可以通过受损的细胞膜进入到死细胞内并嵌入细胞的DNA双螺旋从而产生红色荧光,因此死细胞会被检测到红色荧光。除了用荧光显微镜外,也有报道可以用流式细胞仪和荧光酶标仪来进行定量检测。
荧光特性
Calcein-AM : λex=490 nm , λem=515 nm
PI : λex=530 nm , λem=580 nm
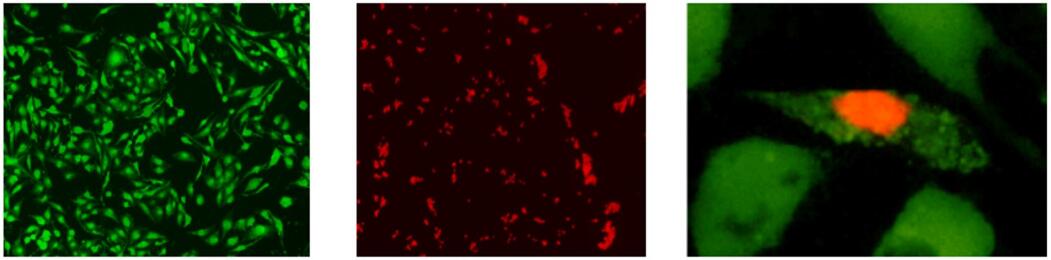
染色例
细胞染色实例
(a) (b) (c)
a) Calcein-AM染FTC细胞(活细胞单染)
b) PI染HCT116细胞(死细胞单染)
c) Calcein-AM、PI染MHD-1 细胞(活死细胞双染)
最佳浓度摸索
由于不同细胞种类、细胞浓度的染色条件不同,我们建议自行摸索一下Calcein-AM和PI的最适浓度。
Calcein-AM和PI的最佳浓度是根据不同的细胞种类而定,通过以下的操作,我们可以找到不同细胞染色试剂的最佳浓度:
1. 通过在0.1%皂苷或0.1-0.5%毛地黄皂苷中培养10 min或通过在70%乙醇中培养30 min制备死细胞。
2. 用0.1-10 μM PI溶液染死细胞,以便找到仅针对细胞核染色而不对细胞质染色的PI浓度。
3. 用0.1-10 μM Calcein-AM溶液染死细胞,以便找到不对细胞质染色的Calcein-AM浓度,再以此浓度的Calcein-AM对活细胞染色以检验活细胞是否被染色。
操作步骤
以HeLa细胞为例
制备1 mmol/l的Calcein-AM储存液
【500 次】
将200 μl DMSO加入到含200 μg Calcein-AM粉末的管中,用移液器吹打溶解。
【3000 次】
将1 ml DMSO加入到含1 mg Calcein-AM粉末的管中,用移液器吹打溶解。
※Calcein-AM储存液需要避光,在-20℃密封保存。
制备染色工作液
将Calcein-AM储存液和PI储存液恢复至室温后使用。
在5 ml的PBS(-)中加入10 μl Calcein-AM储存液和15 μl PI储存液,混匀制成工作液。此时Calcein-AM的浓度为2 μmol/l,而PI的浓度为4.5 μmol/l。
染色步骤
1、 用Trypsin-EDTA消化细胞。
2、 通过离心收集细胞(1,000 rpm,3 min)。
3、 去除上清液,加入PBS(-)制备细胞悬液(105 – 106 cells/ml为宜)。
4、 重复步骤2和步骤3数次以消除培养基中的酯酶活性。
5、 取100 μl 染色工作液与200 μl 细胞悬液混合,在37℃培养15 min。
6、 在490±10 nm激发波长下同时观察黄绿色荧光的活细胞和红色荧光的死细胞。另外用545 nm激发波长单独观察死细胞。
注意事项
1、 由于本试剂盒中的Calcein-AM Reagent 粉末和PI Stock Solution量很少,有可能会粘在盖子或管壁上,开封前请先涡旋以使其振落下来。
2、 由于Calcein-AM储存液对潮气敏感,请在使用后密闭Calcein-AM储存液的盖子。如果不能一次用完,建议分装保存,例如分装成10 μl/管,用封口膜封口,并用铝箔纸包裹,放在一个密闭性能好的塑料袋中,并放入一包干燥剂,在≤-20℃密封避光保存。
3、 配制好的染色工作液请在当天使用。
4、 PI有疑似致癌性,使用前应注意以下几点:
1) 使用时请带好手套,口罩,防护眼镜等,不要接触到或呼吸到。
2) 当PI不慎接触到皮肤时,请立刻用大量的水冲洗。
3) 处理方法
清洗容器的清洗液和废液请按照实验室的有毒有害物质的处理方法进行处理,或按照以下方法处理:
・用UV照射的方法进行分解
・用次氯酸钠氧化分解后,进行中和处理
参考文献
1. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration,
Materials Today, 2020, doi.org/10.1016/j.mattod.2019.12.005
2. Mitochondria-Targeted Artificial “Nano-RBCs” for Amplified Synergistic Cancer Phototherapy by a Single NIR Irradiation,
Advanced Science, 2018, 5, 1800049
3. 4D-Printed Biodegradable and Remotely Controllable Shape Memory Occlusion Devices,
Advanced Functional Materials, 2019, 29(51), 1906569
4. Magnetic Hyperthermia-Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Re
generation Bioactivity by 3D-Printing Composite, Advanced Functional Materials, 2019, 1907071
5. A Substitution-Dependent Light-Up Fluorescence Probe for Selectively Detecting Fe3+ Ions and Its Cell Imaging Application,
Advanced Functional Materials, 2018, 28(35), 1802833
6. An Extendable Star-Like Nanoplatform for Functional and Anatomical Imaging-Guided Photothermal Oncotherapy,
ACS Nano, 2019, 13(4), 4379-4391
7. Near-Infrared Light-Triggered Sulfur Dioxide Gas Therapy of Cancer, ACS Nano, 2019, 13(2), 2103-2113
8. Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation,
ACS Nano, 2018, 12(4), 3780-3795
9. Terrylenediimide-Based Intrinsic Theranostic Nanomedicines with High Photothermal Conversion Efficiency for Photoacoustic
Imaging-Guided Cancer Therapy, ACS Nano, 2017, 11(4), 3797-3805
10. Two-Dimensional Graphene Augments Nanosonosensitized Sonocatalytic Tumor, ACS Nano, 2017, 11(9), 9467-9480
11. Multifunctional Bismuth Selenide Nanocomposites for Anti-Tumor Thermo-Chemotherapy and Imaging,
ACS Nano, 2016, 10(1), 984-97
12. Molecular Responses of Human Lung Epithelial Cells to the Toxicity of Copper Oxide Nanoparticles Inferred from Whole
Genome Expression Analysis, ACS Nano, 2011, 5(12), 9326–9338
13. Living functional hydrogels generated by bioorthogonal cross-linking reactions of azidemodified cells with alkyne-modified
polymers, Nature Communications, 2018, 9, 2195
14. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds,
Nature Communications, 2019, 10(1), 2060
15. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered
immunotherapy, Biomaterials, 2019, 219, 119370
16. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration,
Biomaterials, 2019, 207, 61-75
17. Triple-functional polyetheretherketone surface with enhanced bacteriostasis and anti-inflammatory and osseointegrative
properties for implant application, Biomaterials, 2019, 212, 98-114
18. Ultrasmall Cu2-xS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II
biowindow, Biomaterials, 2019, 206, 101-114
19. Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous
NIR irradiation, Biomaterials, 2018, 155, 54-63
20. Cooption of heat shock regulatory system for anhydrobiosis in the sleeping chironomid Polypedilum vanderplanki,
Proc. Natl. Acad. Sci., 2018, 115(10), E2477-E2486
21. Wnt Inhibitor Dickkopf-1 as a Target for Passive Cancer Immunotherapy,
Cancer Research, 2010, 70(13), 5326-36
22. Gadolinium polytungstate nanoclusters: a new theranostic with ultrasmall size and versatile properties for dual-modal MR/CT
imaging and photothermal therapy/radiotherapy of cancer, NPG Asia Material, 2016, 8, e273
23. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics,
Theranostics, 2018, 8(6), 1648-1664
24. Connexin43 Hemichannels Contribute to Cadmium-Induced Oxidative Stress and Cell Injury,
Antioxidants & Redox Signaling, 2011, 14(12), 2427-39
25. Synthesis and characterization of hierarchically macroporous and mesoporous CaO-MO-SiO2-P2O5(M=Mg,Zn,Sr) bioactive
glass scaffolds, Acta Biomaterialia, 2011, 7(10), 3638-3644