特点:
● 只需添加小分子量荧光试剂即可轻松检测线粒体
● 可以使用荧光显微镜进行活细胞成像
● 可以与附着的溶酶体染色剂同时染色


活动进行中
订购满5000元,200元礼品等你拿
线粒体自噬大揭秘丨从实验思路到检测指标 PDF下载
| 关联指标干货参考(点击查看) | 检测指标(点击查看) | |
| 线粒体自噬详述 | Mitophagy Detection Kit(本产品) | |
| 多细胞器共染&线粒体动力学 | MitoBright IM Red for Immunostaining | |
| MitoBright LT Green/Red/Deep Red | ||
| 线粒体功能 | JC-1 、MT-1 | |
| CCK-L、ADP/ATP比率检测 | ||
| Oxygen Consumption Rate(OCR) | ||
| mtSOX | ||
| ROS Assay Kit -Highly Sensitive DCFH-DA- | ||
| ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- | ||
| Ca2+从内质网到线粒体 | Fura 2-AM | |
| Fluo 4-AM | ||
| Rhod 2-AM | ||
| 线粒体自噬-溶酶体功能 | Lysosomal Acidic pH Detection Kit | |
| Lysosomal Acidic pH Detection Kit-Green/Deep Red | ||
| 线粒体自噬-脂质定位&定量 | Lipi-Blue/Green/Red/Deep Red | |
| Lipid Droplet Assay Kit-Blue/Deep Red | ||
| 细胞死亡 | Cell Counting Kit-8 | |
| Cytotoxicity LDH Assay Kit-WST | ||
| Annexin V, FITC Apoptosis Detection Kit | ||
*点击即可跳转至详情页
试剂盒内含
产品概述
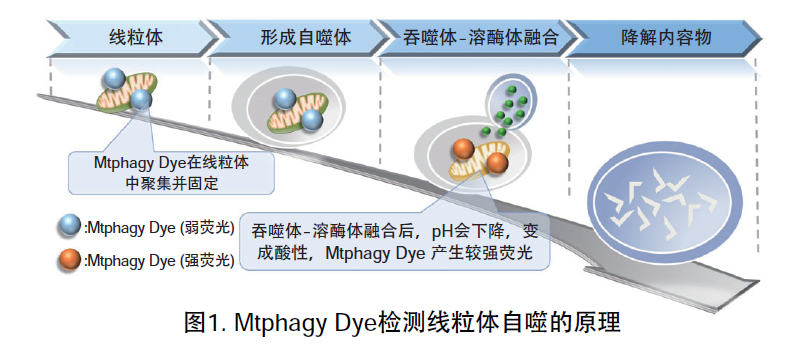
线粒体 (Mitochondria) 是细胞中重要的细胞器之一,可以为细胞活力提供能量 。近年有报道去极化线粒体的积累引起的阿尔茨海默病 (Alzheimer’s Disease) 与帕金森病(Parkinson’s Disease),可能与线粒体自噬有关。线粒体自噬是一种清除机制,可以通过自噬,将氧化应激、DNA损伤因素导致功能失调的线粒体隔离包裹成自噬体(Autophagosome),再与溶酶体 (Lysosome) 融合后降解。本试剂盒内含Mtphagy Dye (用于检测线粒体自噬) 和Lyso Dye (溶酶体染料)。Mtphagy Dye通过化学结合,固定在细胞内的线粒体上,会发出较弱的荧光。当线粒体发生自噬,损伤的线粒体会与溶酶体融合,pH会下降,变成酸性,此时Mtphagy Dye会产生较强的荧光。如想直观观察Mtphagy Dye标记的线粒体和溶酶体的结合,可联合应用试剂盒中的Lyso Dye (标记溶酶体) 进行双染。
特点:
1)只需添加小分子量荧光试剂即可轻松检测线粒体
2)可以使用荧光显微镜进行活细胞成像
3)可以与附着的溶酶体染色剂同时染色
原理
记载了本产品的检测原理和实验例的论文请看MD01论文实验例中第四篇:Live Cell Imaging of Mitochondrial Autophagy with a Novel Fluorescent Small Molecule
实验例
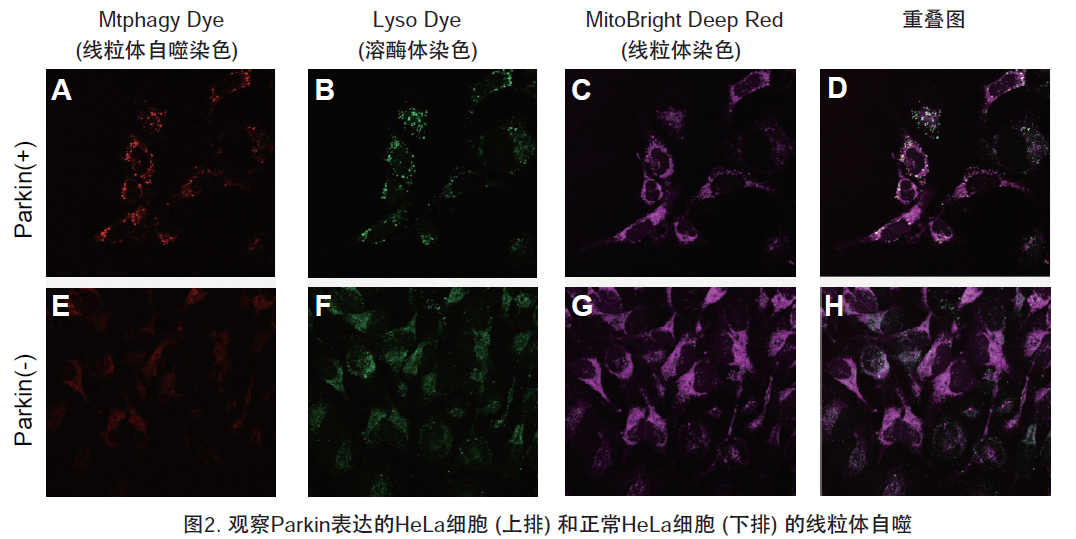
1.用羰基氰化物间氯苯腙 (CCCP,一种线粒体解偶联剂) 诱导Parkin表达的HeLa细胞线粒体自噬,并通过荧光显微镜进行检测。另外,通过与线粒体染色试剂(MitoBright Deep Red:MT08)一同染色,能够区分出已发生自噬的的线粒体(白色)和未发生自噬的线粒体(紫色)(照片:右侧)。
波长:
Mtphagy Dye:561 nm (Ex)、650 LP nm (Em)
Lyso Dye:488 nm (Ex)、502-554 nm (Em)
MitoBright Deep Red:640 nm (Ex)、656-700 nm (Em)
2.荧光显微镜观察
HeLa细胞用CCCP处理,并与线粒体检测试剂(Mtphagy Dye)和线粒体染色试剂(MitoBright LT Green)共同染色,并经过一段时间(6小时)后进行检测。
<检测条件>
设备:LSM-700 Laser scanning confocal microscope (LSCM)
(Carl Zeiss, Oberkochen, Germany)
激发波长:
MitoBright LT Green 488 nm
Mtphagy Dye 555 nm
物镜:63x
拍摄时间:6小时
拍摄间隔:15秒
3.自噬诱导和线粒体膜电位变化关系的检测
用羰基氰化物间氯苯腙(CCCP,一种线粒体解偶联剂)诱导Parkin表达的HeLa细胞线粒体自噬,并使用线粒体自噬检测试剂盒(Mitophagy Detection Kit:MD01)和线粒体膜电位检测试剂盒(JC-1 MitoMP Detection Kit:MT09)观察荧光结果。
结果证实在未经CCCP处理的细胞中几乎未检测到线粒体自噬的发生,并且线粒体膜电位正常维持。 另一方面,在添加了CCCP的细胞中,证实了线粒体膜电位的降低(JC-1的红色荧光降低)和线粒体自噬的发生(Mtphagy染料的荧光增强)。
<实验条件>
■将Parkin质粒导入HeLa细胞
使用HilyMax(货号:H357)将Parkin质粒引入HeLa细胞中(Parkin质粒/HilyMax试剂:0.1 μg/0.2 μl)
过夜培养后进行检测。
■线粒体自噬检测
向表达Parkin的HeLa细胞中添加0.1 μmol/l Mtphagy工作溶液,并在37°C下孵育30分钟。然后将细胞用HBSS洗涤,加入10 μg/ml CCCP/MEM溶液,并在37℃下孵育2小时。在荧光显微镜下观察细胞。
■线粒体膜电位检测
将10 μg/ml的CCCP/MEM溶液添加至表达Parkin的HeLa细胞中,并在37℃下孵育1.5小时。加入4 μmol/l的JC-1工作液使其终浓度达到2 μmol/l,并在37℃下孵育30分钟。孵育后,将细胞用HBSS洗涤,加入成像缓冲液,并在荧光显微镜下观察细胞。
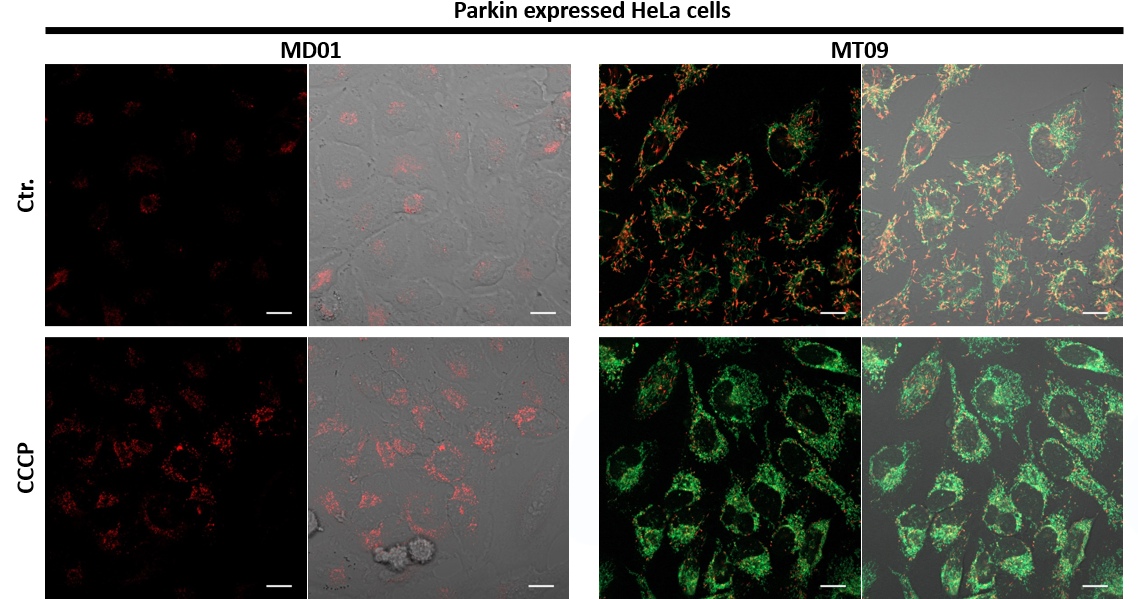
<检测条件>
■线粒体自噬检测
Ex:561 nm,Em:570-700 nm
■线粒体膜电位检测
绿色Ex:488 nm,Em:500-550 nm
红色Ex:561 nm,Em:560-610 nm
荧光特性
参考文献
| 序号 | 检测对象 | 使用仪器 | 文献 |
| 1) | 细胞(HeLa) | 流式细胞仪 | J. Koniga, C. Otta, M. Hugoa, T. Junga, A. L. Bulteaub, T. Grunea and A. Hohna, “Mitochondrial contribution to lipofuscin formation”, Redox Biology, 2017, 11, 673. |
| 2) | 细胞(KB) | 荧光显微镜 | K. Kameyama, “Induction of mitophagy-mediated antitumor activity with folate-appended methyl-β-cyclodextrin”, International Journal of Nanomedicine, 2017, 12, 3433. |
| 3) | 细胞(SH-SY5Y, 初代皮质神经细胞) | 荧光显微镜 | E. F. Fang, T. B. Waltz, H. Kassahun, Q. Lu, J. S. Kerr, M. Morevati, E. M. Fivenson, B. N. Wollman, K. Marosi, M. A. Wilson, W. B. Iser, D. M. Eckley, Y. Zhang, E. Lehrmann, I. G. Goldberg, M. S. Knudsen, M. P. Mattson, H. Nilsen, V. A. Bohr and K. G. Becker, “Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway”, Scientific Reports, 2017, 7, (46208), DOI: 10.1038/srep46208. |
| 4) | 细胞(HeLa、Parkin表达HeLa) | 荧光显微镜 | H. Iwashita, S. Torii, N. Nagahora, M. Ishiyama, K. Shioji, K. Sasamoto, S. Shimizu and K. Okuma, “Live Cell Imaging of Mitochondrial Autophagy with a Novel Fluorescent Small Molecule”, ACS Chem. Biol., 2017, 12, (10), 2546. |
| 5) | 细胞(Cardiomyocytes) | 流式细胞仪 | Y. Feng, NB. Madungwe, CV. da Cruz Junho and JC. Bopassa, “Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy.”, Br. J. Pharmacol., 2017, 174, (23), 4329. |
| 6) | 细胞(HCT116) | 荧光显微镜 | K. M. Elamin, K. Motoyama, T. Higashi, Y. Yamashita, A. Tokuda and H. Arima, “Dual targeting system by supramolecular complex of folate-conjugated methyl-β-cyclodextrin with adamantane-grafted hyaluronic acid for the treatment of colorectal cancer.”, Int. J. Biol. Macromol., 2018, doi: 10.1016/j.ijbiomac.2018.02.149. |
| 7) | 细胞(Parkin-HeLa) | 流式细胞仪 | N. Furuya, S. Kakuta, K. Sumiyoshi, M. Ando, R. Nonaka, A. Suzuki, S. Kazuno, S. Saiki and N. Hattori, “NDP52 interacts with mitochondrial RNA poly(A) polymerase to promote mitophagy.”, EMBO Rep. ., 2018, doi: 10.15252/embr.201846363. |
| 8) | 细胞(NKT) | 流式细胞仪 | L. Zhu, X. Xie, L. Zhang, H. Wang, Z. Jie, X. Zhou, J. Shi, S. Zhao, B. Zhang, X. Cheng and S. Sun, “TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival”, Nature Communications., 2018, 9, (1), doi:10.1038/s41467-018-05097-5. |
| 9) | 细胞(HeLa) | 流式细胞仪 | K. Araki, K. Kawauchi, W. Sugimoto, D. Tsuda, H. Oda, R. Yoshida and K. Ohtani, “Mitochondrial protein E2F3d, a distinctive E2F3 product, mediates hypoxia-induced mitophagy in cancer cells”, Commun Biol., 2019, DOI: 10.1038/s42003-018-0246-9. |
| 10) | 细胞(Bovine Sertoli) | 荧光显微镜 | E. Adegoke, S. Adeniran, Y. Zeng, X. Wang, H. Wang, C. Wang, H. Zhang, P. Zheng and G. Zhang , “Pharmacological inhibition of TLR4/NF-κB with TLR4-IN-C34 attenuated microcystin-leucine arginine toxicity in bovine Sertoli cells.”, J Appl Toxicol., 2019,doi: 10.1002/jat.3771. |
| 11) | 组织(小鼠) | 荧光显微镜 | E. F. Fang, Y. Hou, K. Palikaras, B. A. Adriaanse, J. S. Kerr, B. Yang, S. Lautrup, M. M. Hasan-Olive, D. Caponio, X. Dan, P. Rocktaschel, D. L. Croteau, M. Akbari, N. H. Greig, T. Fladby, H. Nilsen, M. Z. Cader, M. P. Mattson, N. Tavernarakis and V. A. Bohr, “Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease.”, Nat. Neurosci. ., 2019,DOI:10.1038/s41593-018-0332-9. |
| 12) | 细胞(HepG2) | 荧光显微镜 | Iwasawa, T. Shinomiya, N. Ota, N. Shibata, K. Nakata, I. Shiina, and Y. Nagahara , “Novel Ridaifen-B Structure Analog Induces Apoptosis and Autophagy Depending on Pyrrolidine Side Chain”, Biological and Pharmaceutical Bulletin., 2019, 42, (3), 401-410, doi: 10.1248/bpb.b18-00643. |
| 13) | 细胞(U2OS) | 荧光显微镜 | T. Namba, “BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites “, Sci Adv., 2019, 5, (6), 1386. |
| 14) | 细胞(INS-1) | 荧光显微镜 | A. Inamura, S. M. Hirayama, and K. Sakurai, Loss of Mitochondrial DNA by Gemcitabine Triggers Mitophagy and Cell Death’, Biol. Pharm. Bull.., 2019, 42, 1977. |
| 15) | 细胞(HRCEpiC, HRPTEpic) | 流式细胞仪 | Y. Zhao and M. Sun, Metformin rescues Parkin protein expression and mitophagy in high glucose-challenged human renal epithelial cells by inhibiting NF-κB via PP2A activation., Life Sci.., 2020, DOI:10.1016/j.lfs.2020.117382. |
| 16) | 细胞(RAES) | 荧光显微镜 | N. Liu, J. Wu, L. Zhang, Z. Gao, Y. Sun, M. Yu, Y. Zhao, S. Dong, F. Lu and W. Zhang , “Hydrogen Sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high‐glucose and high‐palmitate “, J. Cell. Mol. Med., 2017, 21, (12), 3190. |
| 17) | 细胞(BAECs) | 荧光显微镜 | N. Kajihara, D. Kukidome, K. Sada, H. Motoshima, N. Furukawa, T. Matsumura, T. Nishikawa and E. Araki, “Low glucose induces mitochondrial reactive oxygen species via fatty acid oxidation in bovine aortic endothelial cells”, J Diabetes Investig, 2017, 8, (6), 750. |
| 18) | 细胞(HT22) | 荧光显微镜 | M. Jin, H. Ni and L. Li, “Leptin Maintained Zinc Homeostasis Against Glutamate-Induced Excitotoxicity by Preventing Mitophagy-Mediated Mitochondrial Activation in HT22 Hippocampal Neuronal Cells.”, Front Neurol, 2018, 9, (9), 332. |
| 19) | 细胞(BMDMs) | 流式细胞仪 | D. Bhatia, K. P. Chung, K. Nakahira, E. Patino, M. C. Rice, L. K. Torres, T. Muthukumar, A. M. Choi, O. M. Akchurin and M. E. Choi , “Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis”, JCI Insight, 2019, 4, (23), e132826. |
| 20) | 细胞(U2OS) | 荧光显微镜 | J. Zheng, D. L. Croteau, V. A. Bohr and M. Akbari, “Diminished OPA1 expression and impaired mitochondrial morphology and homeostasis in Aprataxin-deficient cells. “, Nucleic Acids Res., 2019, 47, (8), 4086. |
| 21) | 细胞(HT22) | 荧光显微镜 | D. D. Wang, M. F. Jin, D. J. Zhao and H. Ni, “Reduction of Mitophagy-Related Oxidative Stress and Preservation of Mitochondria Function Using Melatonin Therapy in an HT22 Hippocampal Neuronal Cell Model of Glutamate-Induced Excitotoxicity”, Front Endocrinol (Lausanne), 2019, 10, 550. |
| 22) | 细胞(CD4+T-cells, HeLa) | 荧光显微镜 | A. Bektas, S. H. Schurman, M. G. Freire, A. Bektas, S. H. Schurman, M. G. Freire, C. A. Dunn, A. K. Singh, F. Macian, A. M. Cuervo, R. Sen and L. Ferrucci, “Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy.”, Aging (Albany NY)., 2019, 11, (21), 9234-9263. |
| 23) | 细胞(ALM) | 流式细胞仪 | T. Nechiporuk, S.E. Kurtz, O. Nikolova, T. Liu, C.L. Jones, A. D. Alessandro, R. C. Hill, A. Almeida, S. K. Joshi, M. Rosenberg, C. E. Tognon, A. V. Danilov, B. J. Druker, B. H. Chang, S. K McWeeney and J. W. Tyner, “The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells.”, Cancer Discov., 2019, 9, (7), 919. |
| 24) | 细胞(PK-15) | 荧光显微镜 | Y. Zhang, R. Sun, X. Li and W. Fang, “Porcine Circovirus 2 Induction of ROS Is Responsible for Mitophagy in PK-15 Cells via Activation of Drp1 Phosphorylation”, Viruses., 2020, 12, (3), 289. |
| 25) | 细胞(HCE) | 荧光显微镜 | Y. Huo, W. Chen, X. Zheng, J. Zhao, Q. Zhang, Y. Hou, Y. Cai, X. Lu and X. Jin , “The protective effect of EGF-activated ROS in human corneal epithelial cells by inducing mitochondrial autophagy via activation TRPM2.”, J. Cell. Physiol., 2020, DOI: 10.1002/jcp.29597. |
| 26) | 细胞(心肌细胞) | 荧光显微镜 | Y. Sun, F. Lu, X. Yu, B. Wang, J. Chen, F. Lu, S. Peng, X. Sun, M. Yu, H. Chen, Y. Wang, L. Zhang, N. Liu, H. Du, D. Zhao and W. Zhang, “Exogenous H2S Promoted USP8 Sulfhydration to Regulate Mitophagy in the Hearts of db/db Mice.”, Aging Dis., 2020, 11, (2), 269. |
| 27) | 细胞(HCFs) | 荧光显微镜 | R. Tanaka, M. Umemura, M. Narikawa, M. Hikichi, K. Osaw, T. Fujita, U. Yokoyama, T. Ishigami, K. Tamura and Y. Ishikawa, “Reactive fibrosis precedes doxorubicin-induced heart failure through sterile inflammation.”, ESC Heart Fail., 2020, 7, (2), 588. |
| 28) | 细胞(VSMCs) | 荧光显微镜 | C. Duan, L. Kuang, X. Xiang, J. Zhang, Y. Zhu, Y. Wu, Q. Yan, L. Liu and T. Li, “Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-, BAX-, and GSH- pathways “, Cell Death Dis., 2020, 11, 251. |
| 29) | 细胞(Bovine Sertoli) | 荧光显微镜 | E. O. Adegoke, W. Xue, N. S. Machebe, S. O. Adeniran, W. Hao, W. Chen, Z. Han, Z. Guixue and Z. Peng, “Sodium Selenite inhibits mitophagy, downregulation and mislocalization of blood-testis barrier proteins of bovine Sertoli cell exposed to microcystin-leucine arginine (MC-LR) via TLR4/NF-kB and mitochondrial signaling pathways blockage.”, Ecotoxicol. Environ. Saf., 2018, 116, 165. |
| 30) | 细胞(HeLa) | 荧光显微镜 | D. Takahashi, J. Moriyama, T. Nakamura, E. Miki, E. Takahashi, A. Sato, T. Akaike, K. I. Nakama and H. Arimoto, “AUTACs: Cargo-Specific Degraders Using Selective Autophagy. “, Mol. Cell, 2019, 76, (5), 797. |
| 31) | 细胞(primary hepatocyte) | 荧光显微镜 | H. Kim, J. H. Lee and J. W. Park, “IDH2 deficiency exacerbates acetaminophen hepatotoxicity in mice via mitochondrial dysfunction-induced apoptosis.”, Biochim Biophys Acta Mol Basis Dis, 2019, 1865, (9), 2333. |
| 32) | 细胞(C3H10T1/2s) | 荧光显微镜 | M. S. Rahman and Y. S. Kim, “PINK1-PRKN mitophagy suppression by Mangiferin promotes a brown-fat-phenotype via PKA-p38 MAPK signalling in murine C3H10T1/2”, Metabolism, 2020, 101, 154228. |
| 33) | 细胞(NHEKs) | 荧光显微镜 | S. Ikeoka and A. Kiso , “The Involvement of Mitophagy in the Prevention of UV-B-Induced Damage in Human Epidermal Keratinocytes “, J. Soc. Cosmet. Chem. Jpn., 2020, 54(3), 252. |
常见问题Q&A
|
Q1: 本试剂盒和现存传统方法相比有何优势? |
| A1: 与PH敏感并基于Keima荧光蛋白检测方法相比,本试剂为小分子荧光试剂,因此无需表达荧光蛋白。
另外,可以通过与用于普通活细胞成像的荧光试剂用相同的操作方法对其进行染色和共同观察。 |
| Q2: 使用DMSO配置后的储存液稳定性如何? |
| A2:Mtphagy Dye、Lyso Dye均在制备后需保存在-20℃情况下可以稳定保存1个月。建议按照实验用量,
提前分装保存。 |
| Q3: 工作液稳定性如何 |
| A3: 无法保存,建议现配现用。 |
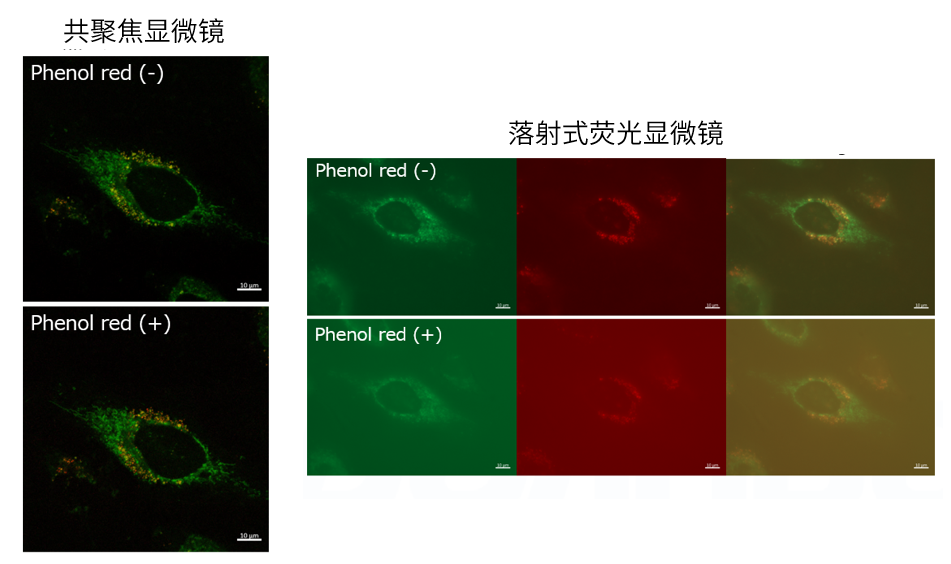
| Q4: 培养基中有酚红会影响检测吗? |
| A4:观察的时候,如果使用共聚焦激光显微镜的话,几乎不会受到酚红的影响,但是使用落射型荧光显微
镜的话,会观察到酚红色的背景。(参照以下观察数据)因此使用落射型荧光显微镜时,请在Working solution进行染色时使用不含酚红的培养基或HBSS。
|
| Q5: 荧光显微镜推荐的滤镜是什么? |
| A5:根据各种试剂推荐以下波长。Mtphagy Dye:激发(500~560 nm)、发射(670~730 nm)
Lyso Dye:激发(350~450 nm)、发射(500~560 nm) |
| Q6:与其他深红色染料共同染色时的注意事项。 |
| A6:Mtphagy Dye比一般的红色系荧光染料相比波长更长,所以和Deep Red的荧光染料一起染色的时候
需要特别注意。即Mtphagy Dye在500–560 nm处激发,可在670-730 nm处检测到荧光,这时与 MitoBright Deep Red的荧光检测波长重叠。因此,有必要在不激发深红色染料的波长下激发Mtphagy 染料,同时在不激发Mtphagy染料的波长下激发深红色染料。 [泄漏的情况] ① 制备仅添加了MitoBright Deep Red(没有添加Mtphagy Dye)的细胞。 ② 通过观察MitoBright Deep Red的激发/发射波长,确认是否观察到荧光(右下图)。 ③ 用Mtphagy Dye的激发/发射波长观察,确认是否观察到荧光(左下图)。 和③中,观察到来自MitoBright Deep Red的荧光(左下图)。 *如果如上所述确认荧光泄漏,请参阅以下内容。 ○调整激发/发射波长 如以上确认如图所示,MitoBright Deep Red也在Ex 561 nm处激发,因此可以将Mtphagy Dye的激发 波长更改为接近激光器或滤光片的500 nm,以使MitoBright深红色不被激发。 调整荧光强度和荧光检测灵敏度 如果MitoBright Deep Red的荧光泄漏到Mtphagy染料的观察波长中,请将观察过程中的激发强度或 灵敏度降低到未观察到荧光的水平。 然后,再确认改变后的观察条件下可以检测Mtphagy Dye的荧光。 [如何检查泄漏] 使用Mtphagy Dye,Lyso dye(溶酶体染色剂),MitoBrightLT Deep Red(线粒体染色剂) 进行三重染色时进行确认 1.在3个培养皿或孔中制备细胞。 (Mtphagy Dye、Lyso Dye、MitoBright Deep Red分别在在不同的皿或孔中进行染色) 2.向每个孔中添加Mtphagy Dye和MitoBright Deep Red。 (在无血清培养基中) 3.在37°C下孵育30分钟。 4.进行自噬诱导条件下(如饥饿培养等)进行培养。 5.向上述2.中未使用的细胞添加Lyso Dye。(在无血清培养基中) 6.在37°C下孵育30分钟。 7.观察每种试剂的激发波长和荧光波长以及荧光强度。 8.检查所用试剂以外的观察波长处的荧光是否没有泄漏。 [观察条件] Lyso Dye:Ex:350-450 nm,Em:500-560 nm Mtphagy Dye:Ex : 500-560 nm,Em :670-730 nm MitoBright Deep Red:Ex :640 nm,Em :656-700 nm |
关联产品