上海金畔生物代理Hampton research品牌蛋白结晶试剂耗材工具等,我们将竭诚为您服务,欢迎访问Hampton research官网或者咨询我们获取更多相关Hampton research品牌产品信息。Products > Crystallization Plates, Hardware & Accessories > 48 Well Crystallization Plates > CrystalHarp Plate (Swissci)
CrystalHarp Plate (Swissci)
Applications
- Capillary diffusion crystallization & in situ X-ray diffraction analysis
Features
- Capillary diffusion offers a broad variable screen in one experiment
- 48 experiments per plate
- in situ diffraction analysis using conventional X-ray sources
- Direct beam line and in situ diffraction analysis possible
- Unique capillary material with easy to remove crystal methodology
- Compatible with any incubation and imaging system
Description
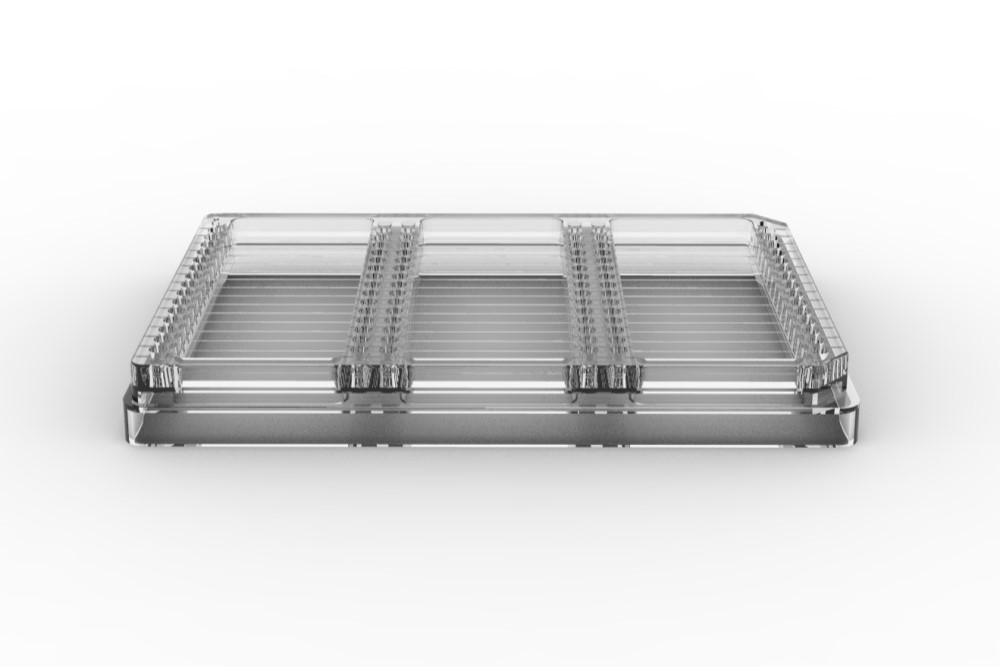
The CrystalHarp plate is designed for crystallization based on capillary diffusion and can be used for crystallization screening and optimization.
Capillary diffusion achieves a much broader screening of variables in one single experiment. The CrystalHarp plate contains 48 capillaries in total and is in an ANSI/SBS 1-2004-standard format to facilitate handling and imaging.
Addition of cryoprotectants or derivatives for phasing studies can be easily added after crystal appearance. The SBS format enables the usage of the plate directly on beam line robots or alternatively single capillaries can be easily mounted to standard magnetic base, enabling in situ diffraction analysis.
The unique capillary material allows data collection at room temperature. Flash-freezing in a liquid nitrogen stream (with or without the use of cryoprotectants) is also feasible. The formation of ice-rings is kept to a manageable minimum.
Contents of Box
1. CrystalHarp plate in shrink wrap
2. Sealant in 10 milliliter syringe with orange delivery nozzle
3. 2 milliliter syringe and flat green nozzle for capillary cleaving
4. Instruction booklet
Swissci product number QH48T-G100
Video demonstration below:
CAT NO
HR3-188
NAME
DESCRIPTION
each
PRICE
$281.00
cart quote
Support Material(s)
 HR3-188 CrystalHarp User Guide
HR3-188 CrystalHarp User Guide HR3-188 CrystalHarp Poster Presentation
HR3-188 CrystalHarp Poster Presentation References
1. A free interface diffusion technique for the crystallization of proteins for X-ray crystallography. F. R. Salemme, Archives of Biochemistry and Biophysics Volume 151, Issue 2, August 1972, Pages 533-539.